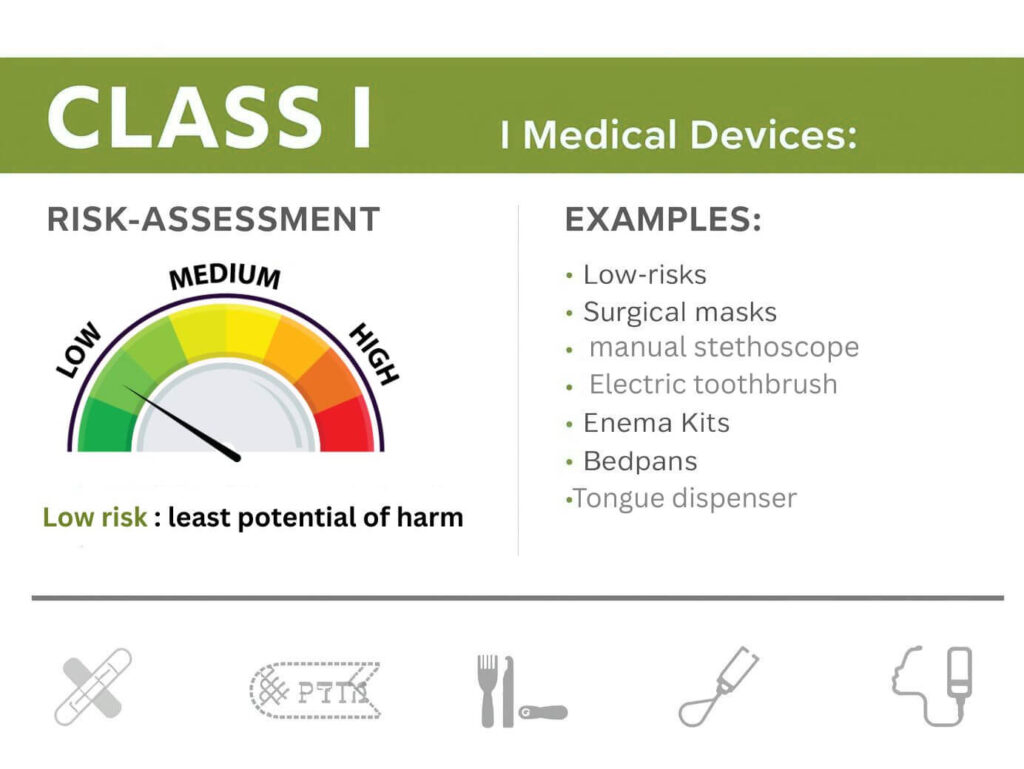
- CDSCO Class 1 Medical Device Registration is necessary to import, manufacture, distribute or sell to the public in India.
- The registration process involves classification, filling out the required forms (MD-3, MD-4, MD-5, MD-14, MD-15), providing DMF/PMF, and site inspection.
- Registration builds compliance, credibility, and consumer trust while enabling wider market access.
- It also ensures adherence to ISO 13485 quality standards and other international benchmarks.
Introduction
A few years ago, a small device manufacturer in Ahmedabad proudly launched a line of non-sterile surgical dressings. The founders assumed that because their devices were “basic” and “low-risk,” the regulatory journey would be quick. But when they filed for CDSCO approval, the application was returned with objections: incomplete Device Master File, wrong classification, and missing details about their plant’s quality system.
What ensued were months of edits, site visits, and document updates. The takeaway? Even for low-risk Class 1 Medical Device, the Central Drugs Standard Control Organization (CDSCO) has a structured process that must be followed. Registration is not a bad piece of paper; it is a process designed to protect patient safety while providing a reputable market and responsible legal pathway.
This guide outlines the entire registration process for Class 1 medical device registration , including the laws, documents, process, and benefits – along with some practical examples and regulatory references.
What is a Class 1 Medical Device?
Class 1 medical devices are those placed in the low-risk category under the Medical Devices Rules, 2017. These are usually non-sterile, non-measuring, and simple medical instruments.
Examples include:
- Adhesive bandages
- Non-sterile cotton swabs
- Manual surgical instruments without measurement functions
- Simple medical dressings
While the risk level is minimal, CDSCO requires these devices to be registered before being imported, manufactured, stocked, or distributed.
“Class 1 medical devices are the simplest in design, but their registration ensures safety and quality at every stage of patient care.”
Some typical devices that fall under Class 1 (non-sterile, non-measuring) include simple bandages, surgical gloves (if non-sterile and basic), certain handheld non-powered instruments. If you have any component involving measurement, sterilisation, or active elements, you may be incorrectly classifying.
How to Determine if a Product is a Class 1 Medical Device
- Read the product label
- Look for FDA approval or classification information.
- Check manufacturer details and safety warnings.
- Check the FDA Medical Device Database
- Recognize device classifications
- Confirm the device’s classification and approval status.
- Class 1 = Low risk, generally exempt from pre-market notification (510(k)) and some GMP regulations.
- Consult a clinician
- Regulatory or clinical experts can help determine classification and safety.
- Contact a qualified manufacturer
- Experts in medical device manufacturing can help with a variety of things such as testing, raw materials, and certification processes.
Common Examples of Class 1 Medical Devices
- Blood pressure monitors (sphygmomanometers)
- Require accuracy and reliability testing; CE mark may be needed.
- Require accuracy and reliability testing; CE mark may be needed.
- Glucose meters (glucometers)
- Must meet ISO 15197 standards for accuracy.
- Must meet ISO 15197 standards for accuracy.
- Infusion pumps
- Deliver fluids or medications; testing required for accuracy and reliability.
- Deliver fluids or medications; testing required for accuracy and reliability.
- Intravenous catheters
- Must minimize infection risk and meet performance standards.
- Must minimize infection risk and meet performance standards.
- Surgical gloves
- Single-use gloves tested for puncture resistance, thickness, and sensitivity (ASTM D3578).
Stepwise Guide to Register for Class 1 Medical Devices
To comply with CDSCO, companies must follow a structured registration process:
- Classify the Device
- Confirm whether the device is Class A (non-sterile, non-measuring).
- Misclassification often leads to application rejection.
- Confirm whether the device is Class A (non-sterile, non-measuring).
- Check Notification Status
- Verify if the device is included in CDSCO’s notified list.
- From October 2023, import licensing became mandatory for all medical devices.
- Verify if the device is included in CDSCO’s notified list.
- Fill the Correct Forms
- Manufacturers use MD-3, MD-4, MD-5, or MD-6.
- Importers use MD-14 and MD-15.
- Manufacturers use MD-3, MD-4, MD-5, or MD-6.
- Prepare Documentation
- Device Master File (DMF) and Plant Master File (PMF).
- ISO 13485 Quality Management System certificate.
- Undertaking of compliance.
- Device Master File (DMF) and Plant Master File (PMF).
- Submit through CDSCO Portal
- Register on CDSCO portal
- Upload forms, documents, and pay fees
- Register on CDSCO portal
- Inspection by Authorities
- State Licensing Authority inspects manufacturing facilities.
- Verifies compliance with PMF, QMS, and hygiene standards.
- State Licensing Authority inspects manufacturing facilities.
- Approval & Licensing
- If compliant, CDSCO issues the license.
- Valid for up to five years with renewal provisions.
- If compliant, CDSCO issues the license.
“Smooth registration depends less on speed and more on accuracy in documentation and classification.”
Legal Framework for Medical Device Registration
The legal foundation of Class 1 medical device registration in India rests on three pillars:
- Drugs and Cosmetics Act, 1940 & Rules, 1945
- The original law regulating drugs and medical devices.
- Covers sale, manufacture, import, and distribution.
- The original law regulating drugs and medical devices.
- Medical Devices Rules, 2017 (MDR 2017)
- Classified devices into Class A (low risk) to Class D (high risk).
- Outlined licensing, inspections, and compliance standards.
- Classified devices into Class A (low risk) to Class D (high risk).
- Medical Devices (Amendment) Rules, 2020
- Expanded scope of regulation.
- Made licensing mandatory for import of all devices, regardless of risk.
- Expanded scope of regulation.
Forms for Class 1 Medical Device Registration
Here are the essential CDSCO forms:
| Form | Purpose | Applicable To |
| MD-3 | Manufacturing license application | Manufacturer |
| MD-4 | Loan license application | Manufacturer (loan basis) |
| MD-5 | Permission to manufacture Class 1 device | Manufacturer |
| MD-6 | Loan license permission | Manufacturer |
| MD-14 | Import license application | Importer |
| MD-15 | Permission to import device | Importer |
Documents Required for Class 1 Medical Device Registration
Documentation is the backbone of the process. You’ll need:
- Constitution details of the manufacturing firm/authorized agent.
- Lease or property agreement for manufacturing site.
- Cover letter explaining application.
- Notarized quality certificate of manufacturing site.
- ISO 13485 QMS certificate.
- Device Master File (DMF).
- Plant Master File (PMF).
- Testing license (if applicable).
- Undertaking of QMS compliance.
- Payment receipt of fees.
- All relevant CDSCO forms (MD-3 to MD-15).
“Even one missing document can stall the registration process for months — detailed preparation saves time and cost.”
Benefits of Class 1 Medical Devices
- Cost-effective
- Affordable, low-maintenance solutions.
- Affordable, low-maintenance solutions.
- High-quality standards
- Must meet FDA or CE safety and performance requirements.
- Must meet FDA or CE safety and performance requirements.
- Safety features
- Designed to prevent errors and enhance user safety.
- Designed to prevent errors and enhance user safety.
- Patient education & monitoring
- Can track health metrics and share information with healthcare professionals.
- Can track health metrics and share information with healthcare professionals.
- Improved procedures and outcomes
- Digital monitoring supports better surgical and treatment results.
- Digital monitoring supports better surgical and treatment results.
- Regulatory Compliance
- Ensures business operates legally in India.
- Prevents penalties, product recalls, or rejection.
- Ensures business operates legally in India.
- Credibility & Trust
- Builds positive reputation among hospitals, distributors, and investors.
- Positions company as compliant and responsible.
- Builds positive reputation among hospitals, distributors, and investors.
- Market Access
- Enables nationwide and international distribution.
- Many foreign partners prefer CDSCO-compliant suppliers.
- Enables nationwide and international distribution.
- Customer Safety
- Assures patients and doctors of safe use.
- Enhances consumer confidence in your brand.
- Assures patients and doctors of safe use.
Classification: Is Your Device Really Class 1?
Correct classification is the first hurdle. Misclassification leads to delays, rejection, or even penalties.
| Class | Risk Level / Description | Who Licenses | Things to Check |
| Class A | Low risk (non-sterile, non-measuring etc.) | State Licensing Authority or as defined under MDR 2017 | Is it non-sterile? Non-measuring? Non-powered? Hand-held / simple? |
| Class B | Slightly higher (measuring / sterility etc.) | SLA or CLA depending on rules | If you add measurement, sensors, etc., maybe B |
| Class C / D | Moderate-high to high risk | Central Licensing Authority (CLA) | More demanding compliance, clinical/performance data etc. |
For “Class 1 medical device registration” (sometimes written “Class 01 medical devices registration,” “Class 1 medical devices registration”) you must ensure your device fits “Class A non-sterile non-measuring” or similar as per current CDSCO notification.
Timeline & Costs
Based on recent regulatory updates and observations from companies:
- Timeline: For Class 1 (low risk) devices, assuming everything is in order, registration might take 2-4 months. But delays from queries or inspection can push it to 5-6 months. For import license (MD-14/15), expect 6-9 months if no issues.
- Fees: Governmental fee for Class A / Class 1 device manufacturing/loan license is less than for higher classes. For example, small licence-fee per site / per device may be required. (Exact amounts depend on whether it is manufacturing vs import vs site vs single device etc.)
Role of CDSCO (Central Drugs Standard Control Organization)

In India, the Central Drug Standard Control Organization (or CDSCO) is the primary regulatory authority for medical devices and is responsible for:
- Classification of medical devices based on risk levels.
- Management of regulatory submissions by manufacturers and importers.
- Site inspections of manufacturers to ensure meeting of standards.
- Issuance of licenses to manufacturers, importers, or marketers of medical devices in India.
CDSCO is responsible for the oversight of medical devices for safety, effectiveness, and regulatory compliance before reaching the market.
Recent Updates & Key Dates to Watch
- From 1 October 2023, import of medical devices under all classes (A/B/C/D) requires an import licence under Forms MD-14 / MD-15. This includes many devices previously exempt.
- Notifications continue to expand or clarify which devices are “notified.” Always check the current CDSCO classification list.
- Rules (2017) and Amendments 2020 are the current legal base; any new gazette notifications may affect classification or process.
Conclusion
Registering a Class 1 medical device in India is not merely a regulatory hoop to jump through, but a regulated process to ensure that even low-risk, medical devices are safe, compliant, and dependable. The CDSCO has been clear that manufacturers and importers must submit the required forms (MD-3 to MD-15), the appropriate documentation (DMF, PMF, ISO 13485), and undergo inspections before approval.
Ensuring proper registration for a business ensures sufficient compliance with the regulatory criteria to avoid recall, lack of legitimacy, decreased market access, and greater arbor meant from customers and stakeholders.
As experienced CDSCO consultants, Diligence Certifications assist with every step of the process—from device classification and documentation to inspections —ensuring your product is fully compliant and market-ready.
Frequently Asked Questions
What is a Class 1 medical device?
Class 1 medical devices include low-risk products like bandages, surgical gloves, and thermometers under the CDSCO authority in India.
Is registration with CDSCO required for Class 1 medical devices?
Yes, you must register with CDSCO to import, manufacture, distribute, or sell Class 1 medical devices.
What forms are required to register as a Class 1 medical device?
There are common forms MD-3, MD-4 MD-5, MD-14, and MD-15 depending on if you are a manufacturer, importer, or distributor.
What documents are needed for registration?
Device Master File (DMF), Plant Master File (PMF), ISO 13485 certificate, compliance documents.
How does CDSCO categorize medical devices?
Devices are classified as Class A, B, C, and D based on risk. Generally, Class 1 devices would be in Class A (low risk)
What is the purpose of a site inspection?
CDSCO conducts inspections to ensure quality systems, manufacturing processes and safety specifications are met.
Are Class 1 medical devices exempt from all regulation?
No. Although Class 1 devices have lighter regulation compared to higher risk devices, they still have to be registered with CDSCO and follow requirements.
What is diligence certification in this process?
Diligence certifications is a verification that your company and device meet CDSCO and international compliance standards and help to increase your chances of being approved.
How long does the registration process take?
The timeline can vary based on the completeness and accuracy of the documents and the time taken by CDSCO to perform the review; and can range from an average only of 3 - 6 months.








 BIS Certification
BIS Certification
 CDSCO
CDSCO
 CPCB
CPCB
 LMPC
LMPC
 WPC Approval
WPC Approval
 Global Approvals
Global Approvals
 TEC
TEC
 ARAI
ARAI
 BEE
BEE
 ISO Certification
ISO Certification
 Drone Registration
Drone Registration
 NOC For Steel
NOC For Steel



















 Business Registration
Business Registration















 Legal Services
Legal Services