Latest updates and announcements from the certification and regulatory compliance world.
Interactive webinars and sessions on certification, standards and regulatory requirements.
Detailed insights and best practices on India’s product certification processes.
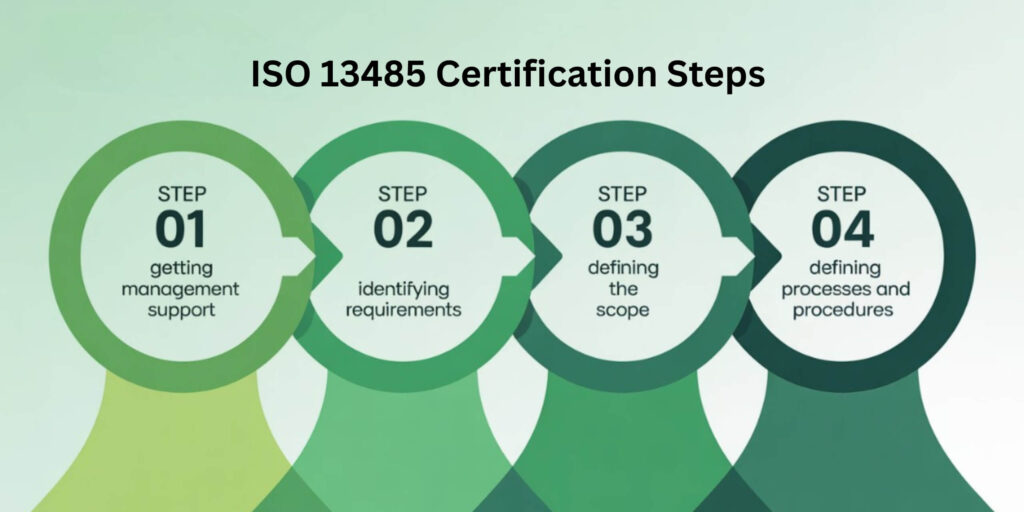
Visual breakdowns of certification steps to make compliance easier to understand.



 BIS Certification
BIS Certification
 CDSCO
CDSCO
 CPCB
CPCB
 LMPC
LMPC
 WPC Approval
WPC Approval
 Global Approvals
Global Approvals
 TEC
TEC
 ARAI
ARAI
 BEE
BEE
 ISO Certification
ISO Certification
 Drone Registration
Drone Registration
 NOC For Steel
NOC For Steel



















 Business Registration
Business Registration














 Legal Services
Legal Services
 Trademark Registration
Trademark Registration
 Copyright Registration
Copyright Registration
 Patent Registration
Patent Registration