- MD-14 is the application form submitted via the CDSCO portal to request permission to import medical devices into India.
- MD-15 is the import license issued by CDSCO after approval of MD-14, granting legal authorization to import specific medical devices for a set validity period (typically 5 years)
- All Class A-D medical devices require CDSCO MD-14 and MD-15 import license, and foreign manufacturers must appoint an authorized Indian agent via Power of Attorney to handle the application.
- Approval process includes document submission, CDSCO review, query resolution, and final issuance
- Compliance obligations post-approval include labeling, record maintenance, post-market surveillance, quarterly/annual reporting, and timely renewal of MD-15 license.
Overview of Medical Device Importation Regulations
India’s medical device industry is flourishing, meaning opportunities exist for new foreign manufacturers. The importation of medical devices into India has specific regulatory requirements, requiring compliance with the regulatory requirements established by the Central Drugs Standard Control Organization (CDSCO). The principal forms that must be managed in this respect will be Form MD-14 and Form MD-15. This document is designed with all the information you will require on the forms needed for the importation license.
In order to attain an import license, in MD-15, to import medical devices, CDSCO requires that an application would be submitted in MD-14 through the online portal. These forms are associated with the Medical Device Rules, 2017. These rules added medical devices into a more sophisticated regulatory framework, which previously was formulated by drugs.
What is Form MD-14?
Form MD-14 is the CDSCO application form used to import medical devices into India. It serves as the initial application document that importers must submit to seek permission to bring medical devices into the country.
Key Features of Form MD-14:
- Application form for requesting import permission
- Must be submitted through the CDSCO online portal
- Required for all classes of medical devices (Class A, B, C, and D)
- Contains detailed information about the manufacturer, importer, and device specifications
- Must be accompanied by comprehensive technical documentation
What is Form MD-15?
A medical device import license is a regulatory approval provided by the CDSCO in order to import medical devices into India. Form MD-15 is the actual import license certificate issued by CDSCO after successful evaluation of the Form MD-14.
Key Features of Form MD-15:
- Official import license certificate
- Issued after approval of Form MD-14 application
- Permits legal importation of specified medical devices
- Valid for a specified period (typically 5 years)
- Device-specific and agent-specific authorization
Who Needs MD-14 and MD-15 Import License?
As of October 1, 2023, importation into India of all Class A measuring/sterile, B, C, and D medical devices will require an MD-14 and MD-15 Import License from the CDSCO.
Entities that are required to obtain MD-14/MD-15:
- Foreign manufacturers: The companies that manufacture the devices outside of India
- Authorized agent: The authorized Indian entity designated by the foreign manufacturer by Power of Attorney
- Import license holder: The appointed agent must hold a license for stock and distribution
- Medical device importers: Businesses that import for distribution, sale, or clinical trial
- Hospitals and other healthcare facilities: If directly importing devices for institution use
An application for an Import License in MD-14 will need to be submitted to CDSCO by an authorized agent who has been appointed by the Power of Attorney.
Medical Device Classification and MD-14 and MD-15 Requirements
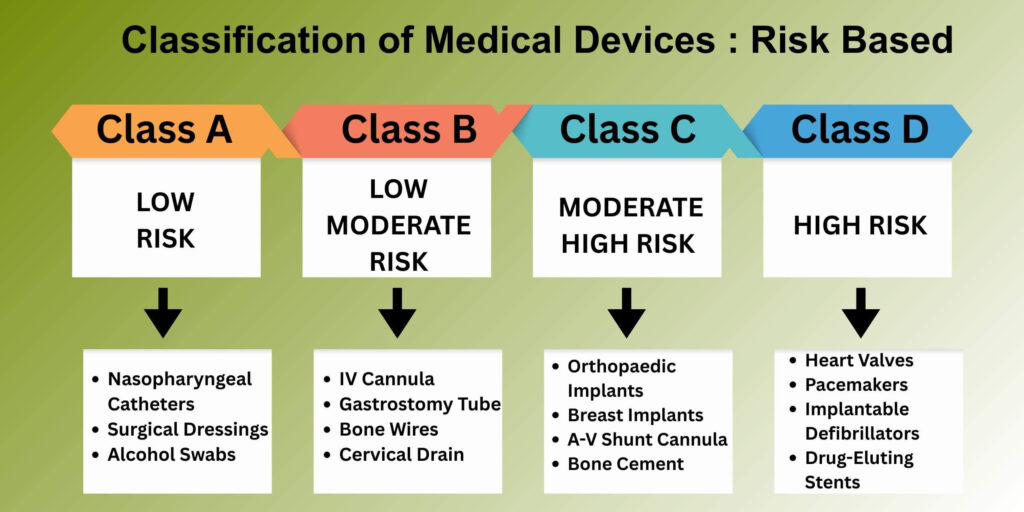
Understanding Medical device classification is crucial as it determines the documentation requirements and approval timelines:
Class A (Low Risk)
- General medical devices with minimal risk
- Examples: Stethoscopes, surgical gloves, bandages
- Simpler documentation requirements
- Faster approval process
Class B (Low-Moderate Risk)
- Devices with moderate risk to patients
- Examples: Surgical needles, syringes, catheters
- Moderate documentation requirements
- Standard approval timeline
Class C (Moderate-High Risk)
- Devices with higher risk potential
- Examples: Orthopedic , ventilators, dialysis machines
- Extensive documentation required
- Longer evaluation period
Class D (High Risk)
- Critical medical devices with significant risk
- Examples: Heart valves, pacemakers, drug-eluting stents
- Comprehensive clinical and technical data required
- Most rigorous evaluation process
Step-by-Step Process to Obtain MD-15 Import License
Step 1: Appointment of Authorized Agent
Foreign manufacturers must first appoint an authorized agent in India through a notarized and apostilled Power of Attorney. The agent must possess:
- Valid MD-42 wholesale license or manufacturing license
- Proper business registration in India
- GST registration
- Physical premises for storage and distribution
Step 2: Device Classification and Documentation Preparation
Determine the device classification and prepare the required documents based on CDSCO guidelines. This includes:
- Device Master File (DMF)
- Product Master File (PMF)
- Technical specifications
- Clinical evidence
- Quality certifications (ISO 13485, CE Mark, FDA approval)
Step 3: Online Application Submission through CDSCO Portal
Access the CDSCO online portal and complete the following:
- Create an account or log in as an authorized agent
- Fill out Form MD-14 with accurate device information
- Upload all required documents in prescribed formats
- Pay the applicable government fees online
- Submit the application and note the application number
Step 4: Document Scrutiny by CDSCO
CDSCO officials review the submitted application and documents for:
- Completeness of information
- Compliance with Medical Device Rules, 2017
- Technical adequacy
- Safety and efficacy evidence
- Manufacturing quality standards
Step 5: Query Resolution (If Applicable)
If CDSCO raises queries or requests additional information:
- Respond promptly with clarifications
- Submit additional documents if required
- Address all concerns comprehensively
- Maintain communication through the portal
Step 6: Approval and Issuance of MD-15 License
Upon successful evaluation:
- CDSCO grants approval
- Form MD-15 import license is issued
- License details are updated in the portal
- Physical or digital certificate is provided
- Importer can legally commence device importation
Essential Documents Required for Form MD-14 Application
- Manufacturing License
- Free Sale Certificate
- ISO 13485 Certificate
- CE Mark or FDA Approval
- Manufacturing Process Flow
- Factory Inspection Report
- Device Master File (DMF)
- Product Master File (PMF)
- Power of Attorney
- MD-42 Wholesale License
- GST Registration
- Address Proof
Timeline for MD-15 License Approval
The approval timeline varies based on device classification and completeness of application:
- Class A Devices: 30-60 days
- Class B Devices: 60-90 days
- Class C Devices: 90-150 days
- Class D Devices: 120-180 days or longer
Factors Affecting Timeline:
- Completeness of initial application
- Quality of submitted documentation
- Number of queries raised by CDSCO
- Responsiveness to clarification requests
- Need for additional testing or clinical data
- Workload at CDSCO office
Validity and Renewal of MD-15 Import License
License Validity Period
The MD-15 import license is typically valid for:
- 3 years for most medical devices
- 5 years for devices with established safety records
- Renewable before expiration
Renewal Process
To renew an MD-15 license:
- Submit renewal application 90 days before expiry
- Provide updated technical documentation
- Submit post-marketing surveillance data
- Pay renewal fees
- Address any changes in device specifications
- Obtain renewed MD-15 certificate
Change of Authorized Agent Process
When changing agents, the new agent must submit all necessary legal documents including MD-14, the new Power of Attorney, government fees, wholesale or manufacturing licenses, label, IFU, and a copy of the import license issued to the former agent, along with an undertaking from the manufacturer.
Change of Agent Requirements:
- New Power of Attorney from manufacturer
- Fresh MD-14 application
- New agent’s MD-42 license
- Surrender of previous agent’s MD-15
- Government fees for agent change
- Manufacturer’s undertaking confirming agent change
- No objection from previous agent (in some cases)
Common Challenges in MD-14 and MD-15 Application
Documentation Issues
- Incomplete Device Master Files
- Missing clinical evidence
- Inadequate risk analysis
- Poor quality translations
- Unsigned or improperly notarized documents
Technical Challenges
- Classification confusion
- Insufficient performance data
- Lack of stability studies
- Missing biocompatibility reports
- Inadequate software validation
Regulatory Challenges
- Changes in CDSCO guidelines
- State-specific requirements
- Import duty and customs issues
- Post-market surveillance obligations
- Adverse event reporting requirements
Agent-Related Issues
- Invalid or expired MD-42 license
- Improper Power of Attorney execution
- GST registration problems
- Inadequate storage facilities
- Lack of qualified personnel
Post-Approval Obligations for MD-15 License Holders
Regulatory Compliance
- Labeling Requirements: All imported devices must have labels in English with:
- Device name and model
- Manufacturer details
- Import license number
- Batch number and manufacturing date
- Expiry date (if applicable)
- Storage conditions
- MRP inclusive of all taxes
- Record Maintenance: Maintain detailed records for 5 years:
- Import invoices
- Batch-wise stock records
- Distribution records
- Customer complaints
- Adverse event reports
- Post-Market Surveillance: Monitor device performance and report:
- Adverse events within 30 days
- Device malfunctions
- Safety concerns
- Patient injuries
- Quality Assurance: Ensure:
- Proper storage conditions
- FIFO inventory management
- Temperature monitoring (if required)
- Regular quality audits
Reporting Obligations
- Quarterly Sales Reports: Submit to State Drug Controllers
- Annual Returns: Provide yearly import statistics to CDSCO
- Vigilance Reporting: Report serious adverse events immediately
- Recall Management: Coordinate with CDSCO for product recalls
Differences Between MD-14 and MD-15
| Aspect | Form MD-14 | Form MD-15 |
| Nature | Application form | License certificate |
| Purpose | Request import permission | Grant import authorization |
| Submitted by | Authorized agent/importer | Issued by CDSCO |
| Status | Initial stage | Final approval stage |
| Validity | One-time application | Valid for 3-5 years |
| Renewal | Not applicable | Renewable before expiry |
Benefits of MD-15 Import License
Legal Authorization
- Legal permission to import medical devices
- Protection from regulatory penalties
- Compliance with Indian laws
- Ability to clear customs without issues
Business Advantages
- Access to Indian healthcare market
- Partnership opportunities with hospitals
- Participation in government tenders
- Enhanced credibility with distributors
Market Expansion
- Entry into growing medical device market
- Distribution across multiple states
- E-commerce platform eligibility
- Brand establishment in India
How to Ensure a Smooth MD-14/MD-15 Licensing Process
Prior to Application
– Engage Regulatory Consultants: Consult with professionals who are experienced CDSCO consultants.
– Understand Device Classification: Make sure you classify the device correctly
– Collect Complete Supporting Information: Prepare the documentation ready for submission.
– Select the Right Agent: Select an agent who holds a valid MD-42 license.
– Verify Technical Data: Make sure the technical files are comprehensive.
During Application
– Use the CDSCO Portal Properly: Follow the instructions for submission you receive when you submit online.
– Keep All Documentation Quality: All documentation must be easy to see and read.
– Respond to Queries from CDSCO Promptly: Respond promptly to any questions or questions initiated from a CDSCO official.
– Check Your Application Status: Constantly check the status of the application.
– Stay in Communication: Always stay in communication via phone, email or in-person visits.
Post Work
– Understand the Terms and Conditions: Understand all terms and conditions available in the license.
- – Prepare for License Expiration: Make sure you are aware of the expiration dates when you need to renew the documentation.
- – Comply with all documents submitted: Record keeping for any import associated with the imported device.
- – Stay Informed: Always review the notifications available on the CDSCO website.
Penalties for Non-Compliance
Operating without a valid MD-15 license or violating import regulations attracts severe penalties:
Financial Penalties:
- Fines ranging from ₹5 lakhs to ₹10 lakhs
- Confiscation of imported devices
- Payment of customs penalties
- Loss of security deposits
Legal Consequences:
- Imprisonment up to 3 years
- Criminal prosecution under D&C Act
- Blacklisting from future applications
- Cancellation of existing licenses
Business Impact:
- Seizure of inventory
- Loss of business reputation
- Termination of agency agreements
- Inability to participate in tenders
Conclusion
Forms MD-14 and MD-15 constitute more than just a requirements – they are your entry point into India’s fast growing medical device market. The application process may appear complicated, however thorough planning, adequate documentation, and following the CDSCO processes will allow for a smooth approval process.
By recognizing the intricacies of the MD-14 process and adhering to MD-15 compliance, a foreign manufacturer may become a player in India. The salient point is to partner with qualified authorized representatives, consult experienced regulatory consulting firms, and remain dedicated to quality and performance standards.
As India’s medical device regulatory framework continues to mature, being informed with CDSCO regulatory updates and remaining compliant is paramount to establishing a thriving medical device business in India.
Ready to import medical devices ? Start your MD-14/MD-15 application today through the CDSCO portal and unlock the potential of India’s healthcare revolution!
Frequently Asked Questions (FAQs)
Can I import medical devices without MD-15 license?
No, importing medical devices without a valid MD-15 license is illegal and attracts severe penalties including fines and imprisonment.
How long does MD-15 approval take?
Approval timelines range from 30 days for Class A devices to 180 days or more for Class D devices, depending on application completeness.
Can one MD-15 license cover multiple devices?
Yes, you can apply for multiple devices under a single application, but each device must be separately listed and documented.
What happens if my MD-15 license expires?
Expired licenses make further imports illegal. Apply for renewal 90 days before expiry to avoid business disruption.
Can I change my authorized agent after obtaining MD-15?
Yes, but you must submit a fresh MD-14 application with the new agent's details and obtain CDSCO approval for the change.
Is MD-15 license required for devices used in clinical trials?
Clinical trial devices require Form MD-27 permission instead of MD-15, but commercial import post-trial requires MD-15.
What is the difference between MD-15 and MD-42 license?
MD-15 is an import license for bringing devices into India, while MD-42 is a wholesale license for distributing devices within India.
Can I apply for MD-14/MD-15 myself or do I need a consultant?
While you can apply yourself, engaging experienced regulatory consultants significantly increases success rates and reduces approval time.








 BIS Certification
BIS Certification
 CDSCO
CDSCO
 CPCB
CPCB
 LMPC
LMPC
 WPC Approval
WPC Approval
 Global Approvals
Global Approvals
 TEC
TEC
 ARAI
ARAI
 BEE
BEE
 ISO Certification
ISO Certification
 Drone Registration
Drone Registration
 NOC For Steel
NOC For Steel



















 Business Registration
Business Registration















 Legal Services
Legal Services
 Trademark Registration
Trademark Registration
 Copyright Registration
Copyright Registration
 Patent Registration
Patent Registration

















































