- If you are a manufacturer or an importer looking to sell medical devices in India’s growing healthcare market, the CDSCO medical device registration is crucial (market projected at $200 B in 2023 growing to $370 B in 2025).
- This detailed guide will outline the necessary information about the registration application procedure, device classification, required documents for registration, CDSCO registration timeline , and fees that are incurred and additional best practices that can help achieve application approvals sooner.
- For those companies who are start-ups in India, or are manufacturing firms from another country, it is important to establish an understanding of the applications regarding CDSCO registration to allow legal market access to the manufacture of medical devices in India, and to easily avoid complications and unnecessary costs of delays in registration.
What is CDSCO Medical Device Registration?

The Central Drugs Standard Control Organization (CDSCO) is the national regulator for drugs in India involved with the approval of drugs, medical devices and health products registration & post-marketing. The CDSCO is regulated by the Drug Controller General of India (DCGI) it the Indian government provides oversight in ensuring products registered for sale in India are safe components and quality as regulated by Indian guidelines.
Key Functions of CDSCO:
- Regulatory Compliance: Ensuring all medical devices follow the Indian safety regulations
- Risk Assessment: Categorizing devices according to their risk levels (Class A through D)
- Market Surveillance: Reviewing medical devices’ safety and effectiveness after approval
- International Standards: Harmonizing Indian regulations with international best practices
Why CDSCO Registration is Mandatory ?
CDSCO medical device registration provides several critical benefits:
- Legal Market Access: Only registered devices can be legally manufactured, imported, or sold in India
- Consumer Safety: Registration ensures devices meet safety and efficacy standards
- Market Credibility: CDSCO approval builds trust with healthcare providers and patients
- Regulatory Compliance: Protects manufacturers from legal penalties and market withdrawal
India ranks fourth globally among pharmaceutical suppliers with over 900 manufacturers, making regulatory compliance essential for market success.
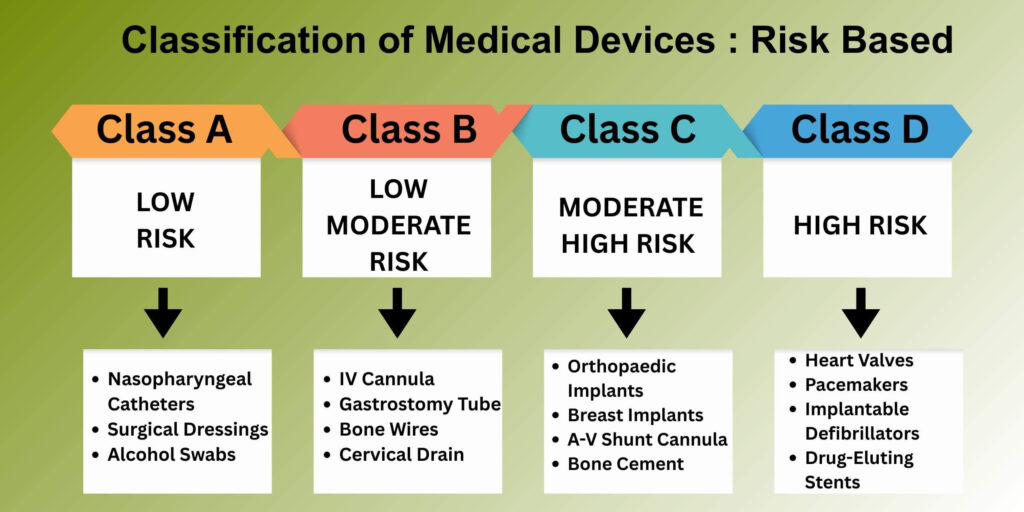
Risk-Based Classification of Medical Devices by CDSCO
CDSCO Classification of medical devices into four categories based on risk assessment, determining the approval pathway and documentation requirements.
Medical Device Classification Table
| Device Class | Risk Level | Examples | Processing Time | Authority |
| Class A | Low | Stethoscopes, bandages, wheelchairs | 3-4 months | State |
| Class B | Low-Moderate | Blood pressure monitors, syringes, thermometers | 4-6 months | State |
| Class C | Moderate-High | Heart valves, orthopedic implants, catheters | 6-9 months | Central |
| Class D | High | Pacemakers, ventilators, heart-lung machines | 9-12 months | Central |
Class A Medical Devices
Considered low-risk, devices that present low potential for patient injury or harm. Examples might include simple medical instruments or equipment that does not penetrate the body or contact with vital organs. Licensing by the relevant state authority may be done with less stringent regulatory review and documentation.
Class B Medical Devices
Considered low-moderate risk that could involve a risk of temporary injury of part of the patient body, should there be equipment malfunctions or misplacement of the device. Examples are non- invasive monitoring, and procedural instruments. These are regulated by state authorities through moderate regulatory review and documentation to ensure the safety and efficacy of the device.
Class C Medical Devices
Considered moderate-high risk where equipment malfunctions or misplacement could cause serious injury or damage to the patient’s body. Devices will have a higher risk for being used where there may be interaction with internal body systems and regulatory authorities will ask for sufficient clinical evidence data of the device within evidence standards. Central licensing authority ensures high expectations for review and safety reviews.
Class D Medical Devices
High risk where devices are life sustaining and can cause death or serious injury should there be an equipment failure. These devices will require most stringent regulatory oversight, comprehensive clinical data, as well as post- market surveillance.
Step-by-Step CDSCO registration process

Step 1: Device Classification and Risk Evaluation
Find the classification of your device according to CDSCO’s classification system:
- Review the Medical Device Rules, 2017
- Check the CDSCO’s device classification database
- Consider the intended purpose of use and how many patients you plan to interact with
- Evaluate the risks and contraindications associated with your device
The primary reason for an application being rejected is incorrect classification. When unable to determine, please use a regulatory expert to classify accurately.
Step 2: Appoint an Indian Authorized Agent (if you are a foreign manufacturer)
Foreign manufacturers must appoint an Indian Authorized Agent or incorporate an Indian company:
Authorized agent responsibilities:
- Submitting documents and answering queries
- Communicating with CDSCO
- Coordinating facility inspections
- Overseeing compliance with procedural requirements post-approval.
- Notifying you of regulatory changes
Selection Criteria:
- Valid drug license
- Regulatory experience
- Geographic proximity to manufacturing sites
- Track record with similar devices
Step 3: Comprehensive Documentation Preparation
Documentation preparation typically takes 3-6 months and requires meticulous attention to detail:
- ISO 13485 Certificate (current and valid)
- Quality manual and procedures
- Free Sale Certificate from country of origin
- Device Master File (DMF)
- Plant Master File (PMF
- Power of Attorney
Step 4: CDSCO Portal Submission
The CDSCO portal streamlines online application submission:
Submission Process:
- Create manufacturer profile
- Select appropriate form (MD-14, MD-15, MD-42)
- Upload documents in specified formats
- Pay applicable fees online
- Submit application with digital signatures
- Receive acknowledgment and tracking number
Common Submission Errors to Avoid:
- Incomplete form fields
- Incorrect document formats
- Missing digital signatures
- Inadequate file sizes
- Wrong form selection
Step 5: CDSCO Review and Assessment
Review Timeline: 90-180 working days depending on device class
Review Process:
- Initial document screening (15-30 days)
- Technical evaluation (30-60 days)
- Query generation and response (30-45 days)
- Final assessment and decision (15-30 days)
Potential Outcomes:
- Approval: License issued with conditions
- Query: Additional information requested
- Inspection: Facility audit required
- Rejection: Application denied with reasons
Step 6: Manufacturing Facility Inspection
Inspection Triggers:
- Class C and D devices (mandatory)
- First-time manufacturers
- Quality system concerns
- Previous compliance issues
Inspection Preparation:
- Update Standard Operating Procedures (SOPs)
- Ensure document traceability
- Prepare quality records
- Train facility personnel
- Conduct pre-inspection audit
A Pune-based device manufacturer achieved zero major observations during inspection by implementing a comprehensive pre-inspection checklist, resulting in immediate license approval.
Step 7: License Issuance and Market Entry
Upon successful review and inspection, CDSCO issues relevant licenses:
License Types:
- MD-5: Manufacturing License (Domestic)
- MD-6: Manufacturing License (Export)
- MD-15: Import License
- MD-17: Import Registration Certificate
Post-Approval Requirements:
- Maintain quality system compliance
- Submit periodic safety reports
- Update product information as needed
- Renew licenses before expiry
Key Documentation for Medical Device Approval
Primary Documentation Categories
1. Quality Management Documents
- ISO 13485 Certificate: Demonstrates quality management system compliance
- Quality Manual: Comprehensive quality procedures and policies
- Manufacturing Licenses: Valid licenses from country of origin
- Facility Certificates: GMP compliance certificates
2. Product Technical Documentation
- Device Master File (DMF): Complete product technical information
- Design History File: Development and validation records
- Risk Management File: ISO 14971 compliant risk analysis
- Labeling and Packaging: CDSCO compliant product information
3. Clinical and Safety Evidence
- Clinical Evaluation Report: Safety and efficacy demonstration
- Post-Market Surveillance: Real-world performance data
- Literature Review: Published clinical evidence
- Biocompatibility Data: Material safety testing results
4. Regulatory Compliance Documents
- Free Sale Certificate: Marketing authorization from origin country
- Certificate of Analysis: Product testing and specifications
- Stability Data: Shelf-life and storage validation
- Software Documentation: For devices with embedded software
CDSCO registration timeline and Costs
Registration Timelines by Device Class
| Phase | Class A | Class B | Class C | Class D |
| Documentation Prep | 2-3 months | 3-4 months | 4-6 months | 6-8 months |
| CDSCO Review | 90 days | 120 days | 150 days | 180 days |
| Inspection (if required) | N/A | N/A | 30-45 days | 45-60 days |
| Total Timeline | 4-6 months | 6-8 months | 8-12 months | 12-18 months |
Cost Structure Analysis
Government Fees
Fees vary based on device class (A, B, C, D) and processing requirements.
Additional Costs
- Foreign manufacturer fee: 25% surcharge
- Multiple manufacturing sites: Additional fees per site
- Inspection costs: Travel and facility preparation
- Consultant fees: Professional regulatory assistance
Hidden Costs to Consider
- Document translation and notarization
- Legal representative fees
- Quality system upgrades
- Clinical study costs (if required)
- Rejection and resubmission costs
License Validity and Renewal
Validity Period
CDSCO medical device licenses are valid for 5 years from issuance date, covering both manufacturing and import licenses.
Renewal Process
Timeline: Submit renewal applications 6 months before expiry
Required Updates:
- Current ISO certifications
- Updated technical documentation
- Compliance with new regulations
- Post-market surveillance reports
- Quality system modifications
Renewal Success Rate: 95% for manufacturers maintaining continuous compliance
Common Challenges and Solutions
Documentation Deficiencies
Problem: Incomplete or non-compliant documentation causing delays
Solution:
- Engage regulatory experts early
- Use CDSCO-specific documentation templates
- Conduct pre-submission reviews
- Maintain document version control
Classification Confusion
Problem: Incorrect device classification leading to wrong approval pathway
Solution:
- Consult CDSCO classification database
- Review similar approved devices
- Seek expert classification guidance
- Document classification rationale
Query Response Delays
Problem: Inadequate query responses extending review timelines
Solution:
- Provide comprehensive initial submissions
- Maintain regulatory expertise
- Respond within specified timeframes
- Address root causes, not just symptoms
Inspection Failures
Problem: Manufacturing facility non-compliance issues
Solution:
- Implement robust quality systems
- Conduct regular internal audits
- Engage inspection specialists
- Maintain continuous compliance
Why Choose Diligence Certifications ?
Reduced Timeline Risks
Professional consultants like Diligence Certifications can reduce approval timelines by:
- 30-40% through optimized documentation
- Minimizing query responses
- Ensuring first-time submission quality
- Coordinating inspection readiness
Cost Optimization
- Avoid rejection and resubmission costs
- Optimize documentation preparation
- Reduce internal resource allocation
- Minimize compliance risks
Regulatory Expertise
- Current regulation knowledge
- CDSCO relationship management
- Best practice implementation
- Strategic planning support
Conclusion
Navigating CDSCO medical device registration requires expertise, attention to detail, and strategic planning. With India’s healthcare market growing rapidly, securing regulatory approval is essential for business success.
Contact Diligence Certifications today for expert CDSCO registration assistance:
- Comprehensive regulatory guidance
- Document preparation and review
- CDSCO portal submission management
- Inspection readiness support
- Ongoing compliance assistance
Don’t let regulatory complexity delay your market entry. Get expert help and ensure successful CDSCO approval.
Frequently Asked Questions
How long will the registration of a medical device with CDSCO take?
The registration time period to obtain CDSCO registration for medical devices will vary by class of the medical device submitted as part of the manufacturer's application: Class A (4 - 6 months), Class B (6 - 8 months), Class C ( 8 - 12 months), Class D ( 12 - 18 months).
3.
What are the charges for publishing a CDSCO registration in 2024 for medical devices?
There will be different CDSCO registration fees depending on the class of medical device submitted in the manufacturing company's application.
Can foreign manufacturers register directly with CDSCO?
Foreign manufacturers cannot register directly with CDSCO, as registration can only be handled by an authorized Indian agent who possesses valid drug licenses or the company must establish an Indian subsidiary with the same registration role as the agent.
What is the difference between notified and non-notified medical devices?
Notified medical devices are formally included in the CDSCO Medical Device Rules 2017 and are required to register. Non-notified medical devices have traditionally been unregulated but are in the process of being systematically regulated and notified.
Is clinical trial data required for every class of medical device?
Clinical trial data requirements differ by class: Class A devices require little or no clinical evidence, Class B devices may require clinical evaluation reports, and Class C and D devices generally require considerable clinical investigation data unless evidence of substantially equivalence is provided.
How do I verify my medical device classification according to CDSCO?
To check the classification of a device, you may refer to the CDSCO classification database, to the device search on the CDSCO portal, or to refer to the Medical Device Rules 2017 in consideration of intended use and risk classification.
Can I import medical devices once the application has been submitted and is in the process as CDSCO registration?
No, you may not import medical devices once the application has been submitted and is in the process within CDSCO registration. All class A-D devices in that case must have Registration and Import license from CDSCO prior to importation.
What will happen if my CDSCO registration application is refused?
If refused, the CDSCO will include detailed reasons in the query letter issued to the applicant. The applicant will have 90 days to address the concerns stated in the letter and submit a new application.








 BIS Certification
BIS Certification
 CDSCO
CDSCO
 CPCB
CPCB
 LMPC
LMPC
 WPC Approval
WPC Approval
 Global Approvals
Global Approvals
 TEC
TEC
 ARAI
ARAI
 BEE
BEE
 ISO Certification
ISO Certification
 Drone Registration
Drone Registration
 NOC For Steel
NOC For Steel



















 Business Registration
Business Registration















 Legal Services
Legal Services
 Trademark Registration
Trademark Registration
 Copyright Registration
Copyright Registration
 Patent Registration
Patent Registration