- Cosmetic Certification guarantees that beauty products coming into India comply with Indian law and safe standards.
- CDSCO Registration is the legal requirement for all cosmetic products imported into India from anywhere (eg, USA, China, South korea).
- The licensing process includes licensed import and export operator, product testing, documentation, and final approval.
- Registration does the following – protects consumers, builds confidence, and allows access to the market without hassle.
- Every importer’s activity is subject to the Drugs & Cosmetics Act, 1940, and, in the case of cosmetics, the Cosmetics Rules, 2020, to stay legal.
Introduction

A Korean skincare company plans to enter the Indian market in 2023 with a distributor in Delhi. They had great sheet masks, serums and creams to sell into a competitive marketplace. However as they were shipping the products into the Indian market they could not clear customs. This is unfortunate but could be avoided. Why? No CDSCO Registration.
This happens often – importers from China, but also the USA and Europe, and more, sometimes have to give up a lot of time, money, energy, and the ability to sell cosmetics in India without the correct writing certification. Cosmetic Certification via CDSCO Registration is so much more than a white NGO formality; it is a way to get into the market, get compliance, and build consumer trust.
Overview of the Indian Cosmetics Market
India’s beauty and personal care industry is growing rapidly. In the past, beauty care was produced by domestic companies but due to urbanization, increased disposable income, and the demand for imported cosmetics of international quality… imported cosmetics are becoming increasingly popular.
- Market Size & Growth: The Indian cosmetics market will grow at a 4.23% CAGR (2022-24) and surpass many (if not most) developed economies.
- Top Importing Countries: India is one of the world’s top importers of cosmetics, especially from China, South Korea, USA, and Europe.
- Consumer Way of Thinking: Indian consumers have evolved into active consumers who check labels, if there are ingredients, and if the certificate is in compliance. They are placing value on the safety of certified products.
Considering the nature of the growth in the beauty industry, the Ministry of Health and Family Welfare has now required CDSCO (Central Drugs Standard Control Organisation) registrations for all imported cosmetics, cosmetic equipment, and associated personal care products to maintain safety and regulations.
What is Cosmetic Certification ?

Cosmetic Certification is the regulatory certification that authorizes imported and locally manufactured cosmetic products for sale in India, and that the products are safe to use by people and are of the defined quality under the Drugs & Cosmetics Act (1940) and Cosmetics Rules (2020).
Certified products:
- Contain no harmful chemicals or microbes
- Are manufactured in compliant facilities
- Are labelled correctly with ingredients, batch numbers and expiry dates
To sum it all up, Cosmetic Certification is your brand’s stamp of legality and ethics – to show safety, quality and consumer confidence.
Why Cosmetic Importers Must Get CDSCO Registration in India
Importing cosmetics into India involves more than just shipping the goods; there are strict laws you must follow under the Drugs and Cosmetics Act, 1940, and the Cosmetics Rules, 2020. All cosmetics products–be it skin care, hair care, or make up–need CDSCO Registration before selling legally. The registration is a verified form of legal use/display, called compliance.
In compliance, not only are consumers protected because of having confirmed that there is no contamination from hazardous substances or microbes, but also benefit your brand with a level of trust with markets, retailers, distributors, and consumers. This certification provides assurance of quality, to ensure acceptable formulations, preservatives, and labels are documented appropriately, while respecting risk of losing whole shipments, fines, taxes, or other legal issues.
In addition to being required by law, as mentioned earlier, major e-commerce and retail chains in India need proof of CDSCO registration prior to selling imported cosmetics. Now, without the registration, you can see how distributing and selling these products in India will not be smooth despite the quality of the products. For this reason, CDSCO registration is compulsory and by definition, not negotiable.
Eligibility to Apply for CDSCO Cosmetic Registration
The following eight entities may apply:
- Authorized Agent: Appointed by a foreign manufacturer to import their cosmetics.
- Indian subsidiary: Branch offices of foreign manufacturers may apply on behalf of the manufacturer.
- Manufacturer: Foreign manufacturers may apply directly as long as they have an authorized agent located in India.
- Independent importers: Any Indian company may import cosmetics as long as it has completed the registration process.
Each application must have complete documentation, and the CDSCO may ask the applicant for more information about its manufacturing processes, ingredient safety, and product stability.
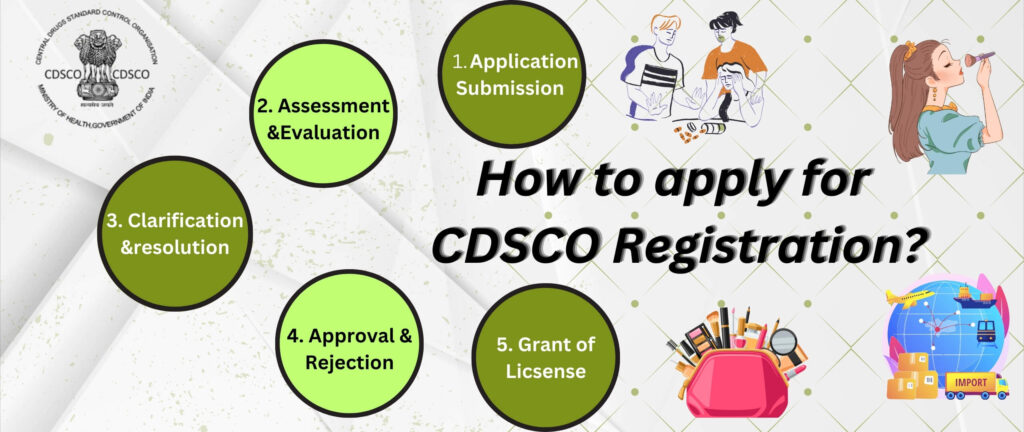
Step-by-Step Guide to Obtaining Cosmetic Certification
Step 1: Identify Product Category & Documentation Requirements
- Imported cosmetics fall into the 80 categories:
- Skin Care – Creams, serums, face masks
- Hair Care – Shampoos, conditioners, dyes, oils
- Oral Care – Toothpaste, mouthwash
- Makeup – Lipsticks, eyeliners, foundations
For each product category, required documents include:
- Product formulation & ingredients
- Manufacturing license of original facility
- Label mock-ups (showing best images of brand name, batch number, expiry date)
- Safety data sheets for chemicals and lab reports.
Step 2: Application Submission (Form COS-1)
Form COS-1 requires you to provide:
- Product description
- Brand name & Pack sizes
- Details of manufacturing location
- Details of preservatives or other chemicals used.
- The importer must also submit the following with their COS-1 form:
- Payment challan in INR
- Certificate of incorporation / business registration
- Quality test reports (if available)
Step 3: Verification & Approval by CDSCO
Upon submission:
- The CDSCO will review documentation.
- The CDSCO will verify claims and check product composition and safety.
- Lab-test reports may be requested and verified.
Upon approval, the CDSCO will issue Form COS-2, issued as a Cosmetic Import Registration Certificate.
This certificate and its accompanying documents must be carried during clearance from customs and produced when requested from customs officials.
Labelling Compliance for CDSCO Certification
Imported cosmetics have strict labelling rules:
- The name and address of the manufacturer/importer
- A batch number and date manufactured
- An ingredient list in descending order by concentration
- An expiry date
- Warnings (for chemicals, allergens or sensitive ingredients)
- Your products may be rejected at customs or may incur fines for non-compliance.
Prohibited Imports
Under the Cosmetics Rules, 2020, the following are prohibited:
- Products banned in their country of origin
- Products with less than 6 months validity at the time of import
- Cosmetics that contain hexachlorophene
- Products that are tested on animals
Costs & Timelines
The expenses associated with obtaining CDSCO Cosmetic Registration can vary based on the number of products, type of cosmetics, and required documentation. Here is an overview:
Application & Documentation
Moderate fees depend on the product category; this includes submitting form and supporting documents to CDSCO.
Product testing
Additional investment may be needed for laboratory testing, safety assessments and stability checks on a per product variation basis.
Consultancy Support
Hiring a regulatory consultant like Diligence Certification can reduce the complexity of the process and avoid delays.
Timeline
Usually takes a few months to half a year, based on the completeness of supporting documents and processing time taken by CDSCO.
Applying early and ensuring all documentation is complete can significantly reduce delays and streamline approvals.
How to Ensure Hassle-Free Cosmetic Import Approval
- Submit registration application before shipping in order to avoid customs hold up
- Update ingredient safety reports database regularly
- Check labels meet Indian requirements
- Be sensitive to expiry dates; imports with <6 months validity will be rejected
- Use experienced CDSCO consultants to minimize documentation hassles
Why Choose Diligence Certification
Understanding CDSCO Cosmetic Registration can feel overwhelming and complicated, especially for first-time importers or brands entering India from abroad. That’s where Diligence Certification comes in. Here is why some of the leading brands count on us:
Experience with the Cosmetic Stream – We have been involved with domestic and international brands for years, helping them with their cosmetic certifications in India.
What does Diligence Certification offer?
- Full Service – We handle everything from preparing the necessary documentation, assisting with Form COS-1 submission, documenting the lab tests, and obtaining CDSCO approvals.
- Time and Money Savings – Our process is structured so that we avoid delays and mistakes, ultimately saving you time and also the risk of incurring penalties.
- Regulatory Assurance – Our priority is ensuring that your imported cosmetics comply with the Drugs & Cosmetics Act, 1940 and Cosmetics Rules, 2020 to minimize shipment rejection risk.
- Individualized Consulting – There is no such thing as a ‘one size fits all’ product. We provide consultations for your specific formulations, variants, and business requirements.
- Think of Diligence Certification as your regulatory GPS – helping steer your products through the Indian cosmetic registration process in a safe, timely, and efficient manner.
Conclusion
The imported cosmetics market in India is extensive, but dealing with the complications and time-consuming nature of CDSCO regulations is not easy. Obtaining Cosmetic Certification through the CDSCO Registration process is much more than a legal requirement and assessment; it enables your imported products to be safe, legally compliant, and available for purchase in every marketplace across India.
Both foreign and Indian importers, whether new to the process or experienced, have the option to obtain assistance from an experienced consultant, which will save them valuable time and money and also expedite the approval process.
Diligence Certifications specializes in guiding brands through the registration process and CDSCO approval through every step of the process (documentation, product testing, packing compliance, labelling compliance, and CDSCO approval), for your imported cosmetics.
With our service support like this, your imported cosmetics could enter into the Indian marketplace quickly and without barriers, be compliant with standards and regulations and assure consumers from day one, that they can trust your product quality.
Avoid unnecessary delays or potential fines, and quickly partner with Diligence Certifications today to simplify your intended importation process of cosmetics and ensure a successful venture.
Frequently Asked Questions
Is CDSCO registration mandatory for imported cosmetics?
Yes. It is required under Drugs & Cosmetics Act, 1940 and Cosmetics Rules, 2020.
Can a foreign manufacturer apply directly?
Yes, through an Indian authorized agent or subsidiary.
Are herbal cosmetics exempted?
No. All cosmetics, including herbal, require CDSCO registration.
Can unregistered cosmetics be sold online?
No. E-commerce platforms require valid CDSCO certification.
Do each product variant or pack size need separate registration?
Yes
What documents are mandatory?
Manufacturing license
Product formula & ingredients
Label mock-ups
Safety and quality reports
What happens if imported products are rejected?
They may be seized, returned, or destroyed.








 BIS Certification
BIS Certification
 CDSCO
CDSCO
 CPCB
CPCB
 LMPC
LMPC
 WPC Approval
WPC Approval
 Global Approvals
Global Approvals
 TEC
TEC
 ARAI
ARAI
 BEE
BEE
 ISO Certification
ISO Certification
 Drone Registration
Drone Registration
 NOC For Steel
NOC For Steel



















 Business Registration
Business Registration















 Legal Services
Legal Services
 Trademark Registration
Trademark Registration
 Copyright Registration
Copyright Registration
 Patent Registration
Patent Registration















































