- Understanding the process of cdsco online registration is critical for firms dealing in drugs, cosmetics, medical devices and related import/export activities.
- The online registration portal by Central Drugs Standard Control Organisation (CDSCO) simplifies compliance by allowing signup, documentation upload and tracking of the application.
- Partnering with experts such as Diligence Certifications ensures compliant, documentation-ready submissions with minimal queries.
- Strategic preparation—such as gathering correct forms, verifying classification of device or drug, liaising with your authorised Indian agent—reduces delays and cost overruns.
- Firms that treat cdsco online registration as a project (with timelines, responsibilities and follow-ups) rather than a tick-box enjoy faster market access and fewer regulatory queries.
Introduction

When a Mumbai–based medical-device manufacturer approached us with ambitions to launch a new diagnostic kit, the single largest hurdle turned out to be their misunderstanding of the cdsco online registration system. They had rushed into producing prototype batches, established export channels, and even negotiated local distributor deals—only to realise that their login to the portal had not been properly activated, the application lacked a valid Indian agent letter, and the classification of the device was incorrectly assumed. After three months and two rounds of query notices, they finally cleared registration—but the delay cost weeks in market access.
For any business in India dealing with drugs, cosmetics or medical devices, the online registration process via the SUGAM Portal (operated by CDSCO) is not just procedural—it determines whether you can legally bring product to market, import stock or run studies such as BA/BE. Mastering cdsco online registration is therefore essential to avoid surprises, wasted effort and regulatory non-compliance.
What is CDSCO Online Registration?
Defining the Process
The term cdsco online registration refers to the process of creating an account and filing an application through CDSCO’s online system (the SUGAM portal) for registration of various regulatory purposes under the Drugs & Cosmetics Act, 1940 and associated Rules.
Regulatory Purpose and Scope
CDSCO is India’s national regulatory body for drugs, cosmetics, medical devices, clinical trials and related activities. Under its umbrella, the online registration serves multiple purposes, including but not limited to:
- Import or manufacturing of drugs (finished formulation or bulk)
- Import registration of cosmetics (including endorsement, new product registration)
- Registration of clinical research organisations (CROs) per the updated rules.
- Medical-device registration or import licensing (while device-specific forms may apply)
Why the Online Route Matters
In earlier days, many applications were manual, paper-based, prone to delays, errors and lack of tracking. The CDSCO shift to the SUGAM portal and the dedicated registration module means:
- applicants can self-register, upload documents, pay fees, track status.
- Queries and discrepancies can be handled online, reducing churn.
- For businesses juggling multiple licences (e.g., import of raw materials, R&D site registration), online registration becomes a single gateway.
- Non-compliance or incomplete registration can lead to major market access delays (as our client story above shows).
Who Must Apply for CDSCO Online Registration?
Identifying Eligible Applicants
If your business touches any of the regulated domains under CDSCO (drugs, cosmetics, medical devices, clinical trials, BA/BE studies), you must consider cdsco online registration. Here is how to break it down:
Drugs/Pharmaceuticals
Any importer, exporter, R&D organisation (such as BA/BE study site), manufacturing unit (for certain categories) must register. According to ClearTax: “Any organisation that imports or manufactures or exports drugs and cosmetics, conducts R&D activities concerning drugs… need to obtain registration under the CDSCO through its registration portal.”
Cosmetics
For companies importing cosmetics into India, or seeking endorsement of existing registration certificates, the online registration process is mandatory. The COS-1 and COS-4 forms process is available online via CDSCO.
Medical Devices & IVDs
Manufacturers or importers of medical devices must check classification (Class A, B, C, D) and then follow the CDSCO process for registration/licensing in addition to online registration. Although “registration” in device sense may refer to later forms (e.g., MD-14/MD-15), the online account creation and submission via portal remains prerequisite.)
Clinical Research Organisations (CROs)
With the recent rule change through the New Drugs & Clinical Trials Rules, 2019 (amended 2024) defining CROs, registration via the online portal is mandatory before conducting human subject trials or BA/BE studies.
Who Does Not Directly Register?
A manufacturer may not register directly—often the corporate entity (holding/parent) creates the login credentials, then the manufacturing unit is associated. ClearTax notes: “Notice – A manufacturing unit cannot register directly on the portal. A corporate needs to create login credentials for the manufacturing unit…”
Thus before you leap into form-filling, clarify which “applicant category” you fall into and whether you are the correct legal entity to apply.
Step-by-Step Guide to Completing the CDSCO Online Registration
Preparing Your Organisation – Pre-Registration Checklist
- Identify the correct Registration Purpose (e.g., “Import of Drugs”, “Manufacture of Cosmetics”, “CRO Registration”) during signup.
- Gather legal entity details: Name, Type (corporate, foreign subsidiary), CIN (if Indian), registered address, contact info.
- Upload identity proof of authorised person, address proof, corporate address proof. ClearTax emphasises these.
- Ensure you have an Indian authorised agent if you are a foreign manufacturer (especially for medical devices/imports).
- Draft an undertaking letter (if required) and ensure digital/mapped signature ready. For cosmetics, a power of attorney may be required.
Registration on the CDSCO Portal
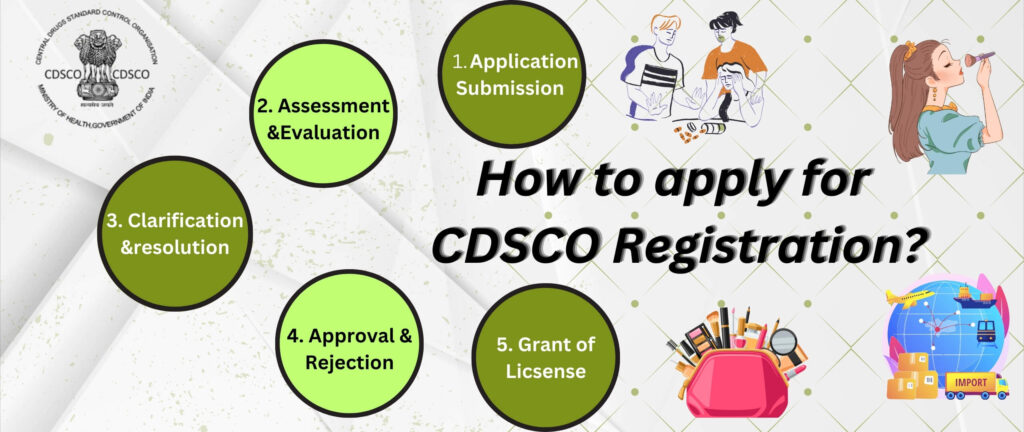
- Visit the portal: “Applicant Registration
- Click “Sign Up Here”, select your Registration Purpose.
- Fill the Applicant Registration form: applicant type, username, password, name, mobile number, email, upload ID proof.
- Submit and verify your email. After approval you’ll receive login credentials.
- Once logged in, you can go to “Add Role” (if you are an existing user needing a new role, e.g., CRO).
Submitting the Main Application
- After login, select the correct form (depending on purpose).
- Fill organisation details, purpose of registration, upload required documents. For example, for cosmetics: covering letter, authorisation letter, ingredient list, label samples.
- For drugs/import: use Form 40 for registration certificate, TR6 challan for fees, power of attorney, manufacturing license or market authorisation certificate.
- Preview the form, edit if needed, then proceed to checklist page (once on checkpoint, no edits allowed) then to payment.
- Make payment via online challan or other modes, upload proof.
- After submission you’ll get a Reference Number — track it on your dashboard.
Review, Queries & Approval
- The concerned CDSCO officer (or designated officer) will review your application. If deficiencies exist, you’ll receive queries via the portal. Example: for cosmetics “Submitted application will be reviewed … If the application is not found satisfactory, then discrepancies … are intimated to the applicant in CDSCO Portal.”
- Respond to queries within the time given, upload missing documents.
- Once all is satisfactory, the registration certificate / import registration number / licence will be generated and can be downloaded.
Timelines & Validity
While CDSCO doesn’t publicly guarantee a fixed timeline for every category, benchmarking suggests:
- For regular online registration (drugs/cosmetics) account and approval may take a few weeks depending on completeness.
- For devices and complex approvals the overall process (registration + licensing) may run into 3-6 months (Class A/B devices) or 6-12 months (Class C/D) as outlined by Qualio.
- Validity of certificate depends on category; for some devices it may be 5 years then renewal required.
Common Challenges & How to Avoid Them
- Incorrect Registration Purpose selected during signup (e.g., “Import of Drugs” vs “BA/BE site”).
- Missing or poorly scanned documents (blurry, illegible, unsigned).
- Label samples not matching product imported.
- Using wrong legal entity (e.g., foreign manufacturer applying instead of Indian authorised agent).
- Lack of tracking and follow-up after submission—queries staying unanswered.
- Underestimating the timeline and launching commercial activity before certificate comes through — this can attract regulatory action.
Avoidance Tips
- Before starting cdsco online registration, convene a checklist review: entity details, agent letter, product classification, label, authorised signatory.
- Allocate one person to monitor the SUGAM dashboard and respond to queries on time.
- Pre-upload high-resolution PDF/PNG versions of all documents to avoid delays.
- If your product is a medical device, engage regulatory expert early to classify device and prepare DMF/PMF.
- Ensure payment of fee is done timely and the challan proof is uploaded immediately.
- Consider keeping a folder of “common queries” (e.g., agent letter not apostilled, label missing batch number) to speed response.
Strategic Considerations for Businesses
Integrate Registration Into Your Launch Plan
Think of cdsco online registration as a business gate-keeper rather than a formality. If your product launch plan assumes entry into the Indian market in six months, allocate the first 2-3 months for regulatory registration, with buffer for queries.
Branding & Market Access Advantage
Having a clean registration certificate from CDSCO not only ensures compliance but also enhances credibility. In the case of our Mumbai client, when they finally received the certificate, they used it in distributor negotiations as evidence of official compliance—this eased distributor risk and improved terms.
Cost of Delay
It’s tempting to shortcut registration, but as noted above, delays can cost weeks of shelf-time, demurrage, lost contracts and legal headaches. Factoring regulatory time into your P&L is prudent.
Document Checklist: What You Must Have Before Applying
Minimum Documents by Category
| Category | Documents Required | Notes |
| Applicant Identity & Address | ID proof (PAN/Aadhaar), Address proof, Corporate address proof | For Indian entity / authorised agent |
| Legal Entity Details | CIN, registered address, contact number | Foreign manufacturers need Indian authorised agent details |
| Product Specific Documents (Drugs) | Covering letter, Power of Attorney, Wholesale/manufacture licence, Schedule D(I)/D(II), GMP certificate or COPP | See guidance document for import/registration of bulk drugs. |
| Product Specific Documents (Cosmetics) | Label sample, Ingredient list, Authorisation letter (agent & manufacturer), Pack sizes & variants | See COS-1 checklist. |
| Product Specific Documents (Devices) | Device classification, DMF/PMF, QMS accreditation (ISO 13485), Clinical evaluation report if needed | See Qualio article. |
| Payment Proof | TR6 challan or other online payment proof | Must upload and reference |
| Online Portal Registration | Signed up via SUGAM, role mapped correctly, e-mail verified | Portal user manual. |
Key Tips
- Scan all documents in one go, name them clearly
- Make sure your label sample shows batch number placeholder and expiry date
- For foreign manufacturer applications: ensure apostille or Indian embassy attestation of POA (if required)
- Retain your reference number from submission; set reminders for next-steps and expiry/renewal
Addressing Timeline & Monitoring Status
Monitoring Mechanism
Once submission is done via SUGAM, you can track status in “Dashboard → My Applications”. Typical statuses: Submitted → Under Review → Query Raised → Resubmitted → Approved. Familiarising with these helps you respond quickly.
Expected Timelines
While no guaranteed time-frame is published, the following guidelines apply:
- Simple cosmetic registration: 4-8 weeks if documents complete
- Drug import registration (finished formulation): may take 2-4 months depending on backlog and queries
- Medical device Class A/B: 3-6 months; Class C/D: 6-12 months or more.
- Setting internal milestones (for your project) keeps the team aligned and avoids surprises.
Renewal & Validity
Many certificates issued by CDSCO have validity of 5 years (especially device licences); renewal must be planned well in advance to avoid lapse. Even online registration part (account) should remain active with updated contact info and role mapping.
Conclusion
Mastering cdsco online registration is as much about preparation as it is about process. For any organisation entering the Indian market, the right regulatory partner makes a measurable difference.
Diligence Certifications offers complete cdsco consultancy—from account creation and document collation to final approval and renewal reminders. Their domain experts track every submission in the SUGAM portal, respond to authority queries, and maintain compliance documentation that stands audit scrutiny.
If your company plans to register products or import under CDSCO, get in touch with Diligence Certifications today to simplify your cdsco online registration journey and ensure a confident, compliant market entry.
Frequently Asked Questions
Who is responsible for controlling the CDSCO online registration process?
The authority that controls the CDSCO online registration process is the Central Drugs Standard Control Organisation (CDSCO) under the Ministry of Health and Family Welfare of India.
Why is CDSCO online registration necessary?
CDSCO online registration is required to safeguard the people of India in ensuring that drugs, cosmetics and medical devices meet India's safety and quality standards before importing into or manufacturing or selling within this country.
Who should apply for CDSCO online registration?
All importers, manufacturers, distributors, and CROs, that work with regulated healthcare products or cosmetics, are required to apply for CDSCO online registration.
How can I apply for CDSCO online registration?
You will first register on the SUGAM portal. Then, you select your category for the regulations, fill in the various forms, upload the corresponding documents, pay the fee, and begin tracking the application status.
How long does CDSCO online registration take?
Timelines may vary; however, for cosmetics it may be around 1-2 months, for drug imports approximately 3 months, and for high-risk medical devices up to 6 months.
What documents are required for CDSCO online registration?
You will need to provide a form of identification, an authorisation letter, a copy of a licence, the product label, test reports, and proof of fee payment.
Is CDSCO online registration relevant for cosmetics?
Yes. If you are an importer of cosmetic products, you must register each product variant under Form COS-1 through the CDSCO SUGAM portal.
Can foreign manufacturers go through the CDSCO registration process without an agent?
A foreign manufacturer completing the registration for CDSCO is unable to do so directly without appointing an agent in India to be responsible for them with content through the online portal.








 BIS Certification
BIS Certification
 CDSCO
CDSCO
 CPCB
CPCB
 LMPC
LMPC
 WPC Approval
WPC Approval
 Global Approvals
Global Approvals
 TEC
TEC
 ARAI
ARAI
 BEE
BEE
 ISO Certification
ISO Certification
 Drone Registration
Drone Registration
 NOC For Steel
NOC For Steel



















 Business Registration
Business Registration














 Legal Services
Legal Services
 Trademark Registration
Trademark Registration
 Copyright Registration
Copyright Registration
 Patent Registration
Patent Registration