- Overview of medical device certification process in India
- Regulatory bodies and Medical Device Rules 2017
- Steps to obtain certification for different device classes
- Required documents and compliance standards
- Benefits of certification for market access and credibility
Introduction

The Medical Device Certification industry in India is expanding rapidly, driven by healthcare technology innovations and the rising demand for high-quality medical products. To maintain safety, efficacy, and conformity with regulations, it is mandatory for medical devices to go through a formal certification process if they are to be produced, imported, or sold in the Indian market. Certification of medical devices in India secures the users of such devices, and at the same time, the manufacturers get the green light from the law and add to their credibility among healthcare providers. Knowing the certification process is a must-have for companies that are willing to make it big in this tightly regulated and competitive industry. Central Drugs Standard Control Organization
Understanding Medical Device Certification
1. Definition of medical device certification
Medical device certification is the formal authorization given by the regulatory bodies, which indicates that a medical device complies with the safety, quality, and performance standards that have been set. This is a guarantee that the device is safe for use and that it works as it is supposed to.
2. Why certification is mandatory in India
India makes it compulsory for medical devices to be certified in order to safeguard the patient’s health, guarantee the quality of the product, and follow the law as per the legal framework laid down by the Central Drugs Standard Control Organization (CDSCO). Simply put, a medical device manufacturer does not have the right to produce, bring in, or market a device in India without first obtaining the required certification from CDSCO.
3. Difference between certification and licensing
Certification is an official confirmation that a medical device complies with the set standards, whereas a license is a permission to manufacture or sell the device. Certification is for a product, but licensing is for a company that makes or imports the product. Both are necessary milestones on the way to compliance with the regulations.
Importance and Benefits of Medical Device Certification
| Importance | Benefits |
| It is a must for the devices to be safe for patients and reliable in their functions. | Confidently healthcare providers and patients trust the devices. |
| Conformity with Indian regulatory requirements. | A formal legal permission to manufacture, import, and sell devices. |
| Product recalls and liabilities are minimized. | Opportunity for a competitive advantage in local and foreign markets. |
| It is a great support to quality management and standard processes. | Obtaining medical device certification makes it easy for the business to access the market and grow. |
| Safety standards notwithstanding, it is possible to keep innovating. | An improved brand reputation and credibility. |
Step-by-Step Process for Medical Device Certification
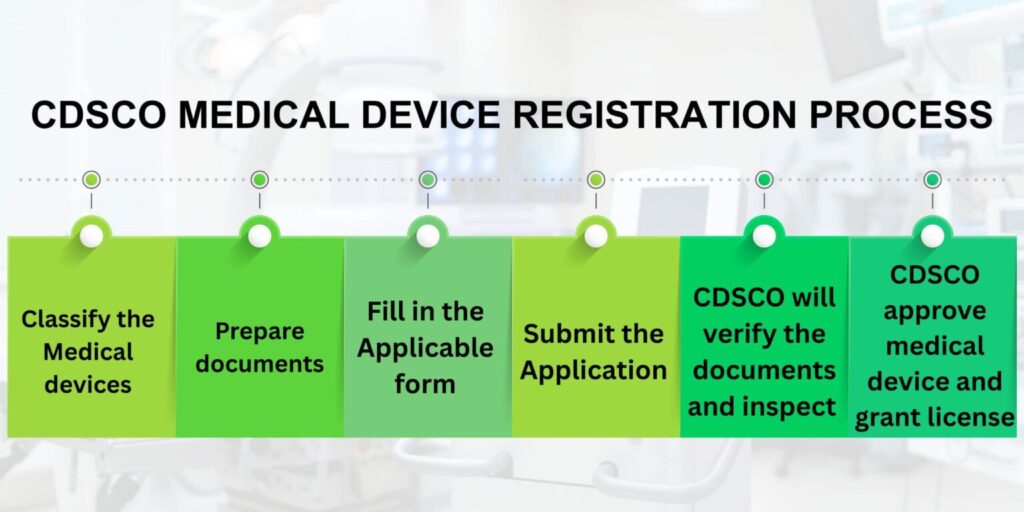
Step 1: Determine device classification
- In the very beginning, it is necessary to figure out the kinds of medical devices according to the Medical Device Rules, 2017.
- Subsequently, the devices are to be classified as Class A, B, C, or D based on the degree of risk they associate with.
- Besides that, a person may check the regulatory approvals of similar devices and also see if there are already some specific directions or waivers available for the devices concerned.
- Make sure that special guidelines or exemptions apply.
Step 2: Prepare necessary documents
- Start with the compilation of a technical file that would be a detailed description of the device design along with its specifications.
- Afterward, furnish the file with Clinical Evaluation Report and relevant clinical trial data.
- Download the safety management reports prepared according to ISO 14971.
- The most important thing is that labeling, packaging, and user manuals shall comply with the requirements set out by the regulatory authorities.
Step 3: Apply for Import License/Manufacturing License via CDSCO
- Registration of the manufacturer or the importer on the CDSCO online portal is the very first thing that should be done.
- Along with it, one is to submit all the required documents and the details of the device classification.
- By paying the necessary fees, an applicant completes a license application.
- From the time of submission to the time of approval, one can follow up on his application through the status of his application and respond to any inquiries from CDSCO without delay.
Step 4: Conduct testing and quality assessment
- Device testing should be done in accredited laboratories to prove its safety and performance.
- At the same time, a manufacturer needs to implement the ISO 13485 quality management system standards and at last qualm the auditors’ doubts by providing documented evidence that all materials and components used meet regulatory specifications.
- Besides, testing results, as well as any corrective action taken, should be recorded and kept ready for inspection.
- Document test results and corrective actions, if any, for audit purposes.
Step 5: Inspection and audit by authorities
- Proper preparation of the manufacturing facility will help ease the burden of a CDSCO inspection.
- Taking down notes regarding processes and quality systems will also prove helpful in this endeavor.
- What is more, the facility staff should be ready to talk with auditors when they come to evaluate the site.
- Furthermore, an inspection report’s mention of non-conformities should be the first step in correcting them.
Step 6: Certification approval and issuance
- CDSCO certification and licensing will be complete with the submission of closing reports and responses to the audit.
- Official certification or license approval will be granted to the holder by the authorities after this stage.
- The certificate validity period should be in the days, weeks, or months depending on the case.
- Compliance has to be maintained in order to be able to renew the license prior to its expiration date.
Documents Required for Medical Device Certification
| Document | Purpose / Description |
| Technical File | Details device design, specifications, and functionality. |
| Quality Management System Certificate (ISO 13485) | Ensures compliance with quality standards in manufacturing. |
| Clinical Evaluation Report | Provides clinical data proving safety and efficacy. |
| Risk Management Report (ISO 14971) | Identifies and mitigates potential risks associated with the device. |
| Labeling and Packaging Information | Ensures correct usage instructions, warnings, and compliance with regulations. |
| Device Master File | Comprehensive document covering materials, components, and manufacturing process. |
| Regulatory Forms | Forms required by CDSCO for license application and registration. |
| Declaration of Conformity | The manufacturer declares that the device meets regulatory and safety standards. |
Role of ISO Standards in Medical Device Certification
ISO standards play a crucial role in ensuring that Medical Device Certification in India meet global quality, safety, and performance expectations. These standards help manufacturers establish strong quality systems, reduce risks, and streamline regulatory approvals under CDSCO. Here’s a clear explanation of their role:
- ISO 13485 defines a quality management system that is tailored to medical device manufacturing thus ensuring the same product quality is maintained.
- ISO 14971 offers a comprehensive framework for the identification, assessment, and control of risks resulting from the entire life cycle of the device.
- ISO standards are the foundation for compliance with Medical Device Rules 2017 in India, thus certification becomes easier and more expected.
- By using ISO standards, the devices produced in India will be accepted in more countries, thus the companies will be able to enter the global market.
Why choose Diligence Certifications
- Firstly, they are highly skilled in all aspects related to CDSCO regulations, device classification, documentation, and ISO standards, thus being able to lead you out of the forest of compliance mistakes.
- Secondly, they are available for you 24/7 and are thus providing you with a truly end-to-end service starting with documents preparation, going through licensing, testing coordination, and finishing audit assistance.
- Thirdly, their knowledge and skills help them to be very effective in approval processes and thus, they are usually able to reduce your approval period and consequently, your time-to-market is improved.
- Lastly, they always keep an ear to the ground when it comes to medical device regulations and hence, your certification process will always be in line with the newest requirements.
Conclusion
Medical Device Certification is the basis of product safety in India, a prerequisite essential to access the healthcare market in a simple way as well as satisfying the requirements of the regulations. Compliance with CDSCO requirements and use of international standards like ISO 13485 and ISO 14971 enable manufacturers to make devices that are safe and of quality that are acceptable both at home and abroad. Moreover, you can accomplish the whole method more efficiently, thus relieving you if you hire the support of an established company like Diligence Certifications that provides you with the utmost convenience throughout the entire process.
Frequently Ask Questions
What is medical device certification?
Medical device certification is basically a formal permission, which acknowledges that the device complies with safety, quality, and performance standards.
Is certification mandatory in India?
As per the Medical Device Rules 2017, all medical devices require certification.
Who regulates medical device certification in India?
The central drugs standard control organization (CDSC0) is the chief executive of the certification authority.
How are medical devices classified?
Devices are divided into four classes A, B, C, or D, which are distinguished based on the degrees of risk.
What is the difference between certification and licensing?
Certification refers to a product being marked with a green light; licensing is the provision of legal authority to a company to manufacture or sell devices.
What documents are required for certification?
The first and most important documents are technical files, clinical reports, risk management, labeling, and ISO certificates.
What role do ISO standards play?
ISO standards such as 13485 and 14971 serve as medical device quality management and risk sources.
What are the main steps in the certification process?
The main steps in the certification processes, in short, would be: Device classification → Documentation gathering → Application submission to CDSCO → Testing → Inspection → Certification approval.
What are the benefits of certification?
Chief among the benefits of certification are patient safety, legal conformity, market accessibility, and credibility enhancement.
Can a consultancy help in certification?
Indeed, a team of professional consultants can undoubtedly ease the process of documentation, audits, and obtaining approval whereby they not only shorten the time taken but also render the process error-free.








 BIS Certification
BIS Certification
 CDSCO
CDSCO
 CPCB
CPCB
 LMPC
LMPC
 WPC Approval
WPC Approval
 Global Approvals
Global Approvals
 TEC
TEC
 ARAI
ARAI
 BEE
BEE
 ISO Certification
ISO Certification
 Drone Registration
Drone Registration
 NOC For Steel
NOC For Steel



















 Business Registration
Business Registration














 Legal Services
Legal Services
 Trademark Registration
Trademark Registration
 Copyright Registration
Copyright Registration
 Patent Registration
Patent Registration
















































