- CDSCO Certification is compulsory to manufacture, import or sell drugs, cosmetics or medical devices
- The CDSCO is a certification issued by the Central Drugs Standard Control Organization, ensuring compliance with a Drugs & Cosmetics Act, 1940.
- There are different types of licenses: Drug License,Cosmetic License and Medical devices
- Applications are submitted through the CDSCO portal – you will need to have documents such as GMP certificates, site master files, and Free Sale Certificates.
- There are consulting firms like Diligence Certifications that can help your business avoid delays, rejection, and compliance issues.
- A cosmetic license (Form COS-1) is required for importers and brands.
- Medical devices are classified from Class A to D, and each class needs specific registration forms.
Why Market Entry Starts with CDSCO Certification
Two years ago, a small Ayurvedic skincare brand from Kerala tried to launch a herbal night cream across India. Their formulation was safe, packaging attractive, and demand from retailers was strong. But when they attempted to scale, distributors asked one simple question: “Do you have CDSCO approval?”
Without it, retailers refused to stock their product. After six months of delays, penalties on imports of raw materials, and a regulatory warning, the founders finally approached a compliance consultant. Within three months of proper CDSCO registration, their cream legally entered the shelves of big chains like Apollo Pharmacy.
This story isn’t rare. Many businesses underestimate CDSCO certification until regulations block their path. That’s why understanding it deeply is crucial—whether you are a drug manufacturer, cosmetic importer, or medical device company.
What is CDSCO Certification?
CDSCO Certification is the legal authorization given by the Central Drugs Standard Control Organization (CDSCO) under the Ministry of Health & Family Welfare allowing you to manufacture, import, distribute, or sell drugs, cosmetics, and medical devices in India.

It ensures an organization complies with:
- The Drugs and Cosmetics Act, 1940
- The Drugs and Cosmetics Rules, 1945
- Updates issued by the CDSCO and Good Manufacturing Practices (GMP).
- Medical Device Rules (MDR), 2017 Specifically governs the regulation of medical devices in India.
Who is required to obtain CDSCO Certification?
- Pharmaceutical manufacturers (allopathy, ayurvedic, veterinary, homeopathy)
- cosmetic brands (domestic and/or imported)
- medical device manufacturers (i.e. syringes, stencils, diagnostics, implants)
- foreign manufacturers entering into India.
To understand CDSCO just think of it as similar to the FDA (food and drug administration) in India; the authority that stands between consumers and unsafe, unauthorized, counterfeit, or uncompromised products.
Why is CDSCO Certification Mandatory?
The Indian market is one of the fastest-growing markets for healthcare and cosmetics in the world. However, without regulation, unsafe formulations will flood the market. The CDSCO is in charge of ensuring three things defined below.
- Consumer Safety: Only tested, effective, and safe drugs and cosmetics are available on the market.
- Legal Compliance: Businesses are forced to close if they operate without a certification.
- International Credibility: A CDSCO approved product makes it easier for export opportunities since CDSCO approval means the product has complied with strict requirements of India.
The reality of doing business without a certification means:
- Imports can be seized at ports
- Manufacturing can be put on hold
- Products can be recalled
- Penalties or jail time can impact owners
What is the Role of CDSCO?
According to the Central Drugs Standard Control Organization (CDSCO), it is defined as “The national regulatory authority for drugs, cosmetics and medical devices in India”. CDSCO works under the Ministry of Health & Family Welfare and is responsible for the registration and approval of new drugs, regulation of imports, licensing of essential drugs – including destination vaccines and blood products, and implementation of the country’s standard quality system.
Manufacturers and importers must obtain CDSCO registration, using the CDSCO portal to register all CDSCO projects (including bioequivalence (BE) and bioavailability (BA)). In other words, CDSCO acts as the gatekeeper to the Indian healthcare market ensuring safe and effective products are available to the consumer.
Types of CDSCO Licenses
CDSCO certification is not one-size-fits-all. Different businesses require different licenses:
1. Drug License
Mandatory for manufacturing, distributing, or selling drugs.
- Retail Drug License (RDL): For pharmacies.
- Wholesale Drug License (WDL): For distributors.
- Manufacturing License: For drug production units.
- Loan License: For companies outsourcing production.
2. Cosmetic License
Covers personal care and beauty products.
- Applies to manufacturers, marketers, and importers.
- Common categories: skincare creams, shampoos, lipsticks, fragrances, hair colours.
- Importers need Form COS-2.
3. Medical Device Registration
Important process for the manufacturers and/or importers of medical devices as required by the Medical Devices Rules, 2017. Devices are classified as Class A (low risk), Class B (Moderate risk), Class C (High risk) and Class D (Critical risk)
Form MD-3: License to manufacture Class A and B devices
Form MD-7: License to manufacture Class C and D devices
Form MD-14/15: For import of medical devices
Forms needed to be submitted included Device Master File (DMF), Plant Master File (PMF), ISO 13485 certification, clinical/performance evaluation reports, and labeling as compliant.
Documents Required for CDSCO Certification
- Application cover letter with company details
- Product specifications
- Packaging label artwork
- Free Sale Certificate
- Power of Attorney
- Site Master File/ Plant master file
- Device Master File
- GMP compliance documents
CDSCO Registration Process
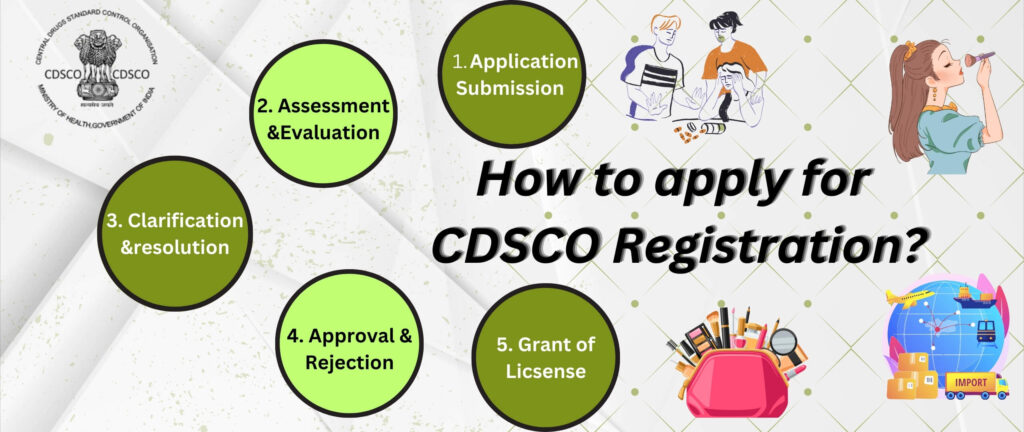
1. Classifying the Medical Device
The first part of the CDSCO registration process involves proper classification of the medical device according to CDSCO regulations. The devices are classified into 4 categories based on risk:
- Class A devices – low risk
- Class B devices – moderate risk
- Class C devices – high risk
- Class D devices – critical risk
Once the device is classified, an application dossier needs to be complied which includes complex and detailed components including:
- the composition and formulation of the device
- the manufacturing process
- regulatory and quality certificates (ISO, GMP or BIS where applicable)
- intended use of device and appropriate labeling
- clinical trial or performance evaluation (if required)
2. Submission of Application to CDSCO Portal
After the application file is created, you can file the application online through the CDSCO portal along with the prescribed fee as stipulated by the government.
In support of the application, you are required to upload all supporting documents for review.
Applicants need to be vigilant to submit all required documents and information in a complete and correct manner, as errors and omissions in the application form usually result in delays in processing.
3. CDSCO Review Process
Once the application is filed, the CDSCO reviews all information provided in order to determine if the drug or device is safe and effective and compliant with regulations.
The review of the application usually includes some combination of:
- a formal review of the data you submitted
- additional questions or clarifications from CDSCO reviewers
- site inspections of the manufacturing facility (domestic or international)
- consultation with technical or clinical experts, if necessary
4. Approval
After consideration, the CDSCO will provide a decision.
Approval: The applicant will be issued the license/registration certificate to manufacture, import or sell the drug or device in India.
CDSCO Certification is the Gate Pass to Indian Markets
Imagine CDSCO certification as having the licence to drive on Indian national highways. Your vehicle (product) must be road-worthy (documentation), pass scrutiny (inspection), and be registered (certificate). Just like driving without certification invites fines and penalties, operating without CDSCO approval invites legal action.
A Pune-based med-tech startup once told us that without CDSCO certification, their FDA-approved ECG monitor was gathering dust in a warehouse—not a sale was possible despite its global pedigree.
Role of Diligence Certifications as Your CDSCO Consultant
At Diligence Certifications, we act as your compliance partner from start to finish.
Our Services:
- Assessing your business model to identify the right license.
- Registering and managing your profile on the CDSCO portal.
- Drafting and reviewing all documentation.
- Liaising with CDSCO officials for faster approvals.
- Representing foreign manufacturers in India.
Why Clients Trust Us:
- 1000+ satisfied businesses across India.
- Expertise in all categories of CDSCO licenses.
- Fast-track strategies for urgent approvals.
- Affordable and transparent pricing.
As one of our clients, a Japanese cosmetic brand, shared:
“We struggled for nearly a year with paperwork until we engaged in Diligence Certifications. Our first import license was cleared in 45 days after their intervention.”
Conclusion
Professional consultants at Diligence Certifications will ensure that your CDSCO application is accurate and complete as well as managed efficiently. From preparing the documentation to directly communicating with regulatory officials, to responding to simple or complex queries, their expertise will help to shorten the waiting periods, and reduce costly delays.
Whether you are launching a new cosmetic brand, importing medical devices, or correctly establishing a pharmaceutical unit, getting a CDSCO Certification is the first and most critical step you will take. A CDSCO Certification is more than just a licence to do business, it offers consumers a seal of safety and access to domestic and foreign markets.
Diligence Certifications offers efficiencies on the entire licensing process to be faster, easier, and reduce the non-productive level of stress that comes with government bureaucracy. When Diligence Certification is your compliance partner, your product can enter the Indian market fully- and legally- with no unnecessary barriers.
Are you ready to get your CDSCO Registration? Call Diligence Certifications today and launch your products with total compliance.
Frequently Asked Questions
What is the difference between a drug license and a CDSCO certification?
A drug license is a permit issued by state authorities to permit retail, wholesale, or manufacturing of drugs. CDSCO certification is an approval that is required at national level for the manufacturing, importing, or distributing drugs, cosmetics, and devices.
Is cdsco certification needed for herbal or ayurvedic products?
Yes. Even ayurvedic, herbal and traditional formulations like, whether for medicinal use or branded as cosmetics, would require licensing under CDSCO. The authority checks the quality and safety of products despite their natural or herbal claim - at the point of sale.
Can a foreign company apply for CDSCO certification directly?
No. Foreign manufacturers must appoint an Indian Authorised Agent to apply for CDSCO certification on their behalf. Applications cannot be processed and will be rejected without a local Authorised Agent (local partners).
How long does it take to get CDSCO certification?
Drug licenses site average around 30 - 60 days, cosmetics licenses average 30 - 45 days, and import licenses average 45 - 90 days. Delays can occur for a number of potential reasons; if documentation is not complete, if CDSCO have queries following an inspection, if further testing is required.
What are the penalties for operating without CDSCO approval?
Operating without a valid CDSCO registration would be in violation of the Drugs and Cosmetics Act, 1940 and could result in a variety of different consequences such as being mandated to recall your goods, seizure of products, financial penalties, and even in some cases, confinement for company officers responsible.
Is the CDSCO license valid indefinitely?
No, CDSCO licenses expire. They are usually valid for a period of 3–5 years depending on license type. You must reapply for a renewal before the expiry date.
Are small startups also required to have CDSCO approval?
Yes. If you are a startup selling cosmetic creams or a startup importing a medical kit. Even a small vendor is still required to have proper CDSCO certification.
What type of fees can I expect in CDSCO certification?
Fees will depend on the type of license; drug license, import license, or cosmetic license. Fees can run from a few thousand rupees to lakhs of rupees depending on how large your product line is. You must also consider lab fees, consultant fees, and renewal fees.
Can a single license cover multiple products?
Usually not. Usually your license will only be for a single product. Each formulation, strength of pill, or cosmetic variants require CDSCO approval. For example, if a company is importing 3 different shampoos, they would apply separately for all three shampoos.
How can consultants speed up the CDSCO approval process?
Professional consultants like Diligence Certification work on your behalf to ensure your documents are correct, will communicate directly with the regulatory officials, and resolve your queries in a very timely manner. With their professional contacts and depth of experience, the processing times will often decrease significantly and help prevent costly delays.



 BIS Certification
BIS Certification
 CDSCO
CDSCO
 CPCB
CPCB
 LMPC
LMPC
 WPC Approval
WPC Approval
 Global Approvals
Global Approvals
 TEC
TEC
 ARAI
ARAI
 BEE
BEE
 ISO Certification
ISO Certification
 Drone Registration
Drone Registration
 NOC For Steel
NOC For Steel



















 Business Registration
Business Registration














 Legal Services
Legal Services
 Trademark Registration
Trademark Registration
 Copyright Registration
Copyright Registration
 Patent Registration
Patent Registration